Visit BYJUS to understand the properties structure and uses of Cellulose C6H10O5n explained by Indias best teachers. Cellulose C6H10O5n - Cellulose is the chemical name of C6H10O5n.

A Level Bond Enthalpy Bond Dissociation Energy Calculations For Enthalpy Of Reaction Ks5 Gce Chemistry Revision Notes
3 S 2019 970104S22 Turn over 2 The feasibility of a chemical reaction depends on the standard Gibbs free energy change Gɵ.

. This is dependent on the standard enthalpy and entropy changes and the temperature. Atomization of methane molecule. For diatomic molecules the enthalpy of atomization is equal to the enthalpy of bond dissociation.
The atomization of. Cs s Cs g H atm 76 KJmol. CH 4 a H 0 16650 kJ mol-1.
The value of enthalpy change is positive because this reaction is endothermic. It is denoted by H atm. Amount of energy required to dissociate 1 mole of the stable molecule into a gaseous atom known as the heat of atomisation.
AState and explain whether the following processes will lead to an increase or decrease in entropy. Moreover q is equal to the standard enthalpy change only when the reactants and products are both at the same temperature normally 25C. Enthalpy Change of Solution.
The irregular trend in the first ionisation enthalpy of the 3d metals though of little chemical significance can be accounted by considering that the removal of one electron alters the relative energies of 4s and 3d-orbitals. Because of the presence of a. Enthalpy of atomisation Transition elements exhibit higher enthalpies of atomization.
It is the enthalpy change when one mole of gaseous atoms is formed from the element. Ithe reaction of magnesium with hydrochloric acid. Enthalpy Change of Atomisation.
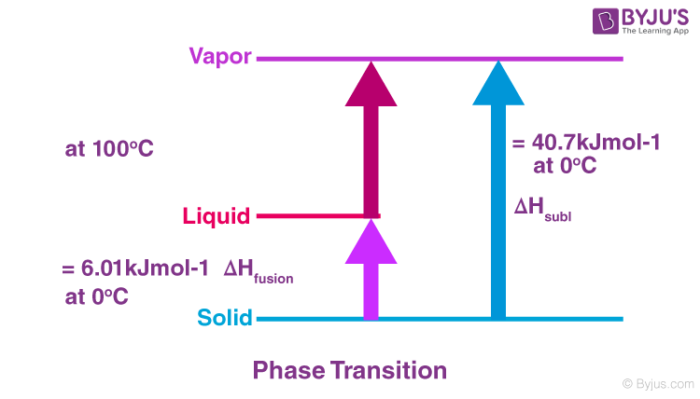
Enthalpy of atomization Δ a H 0 is the change in enthalpy when one mole of bonds is completely broken to obtain atoms in the gas phase. The Heat of Atomisation or Heat of Atomisation.

Caie 9701 11 O N 20 Q 6 Enthalpy Changes Youtube

For Part I How Do I Write The Equation For Atomisation I Remember In Enthalpy Change Of Atomisation One Mole Of Atom Is Formed From Substance That Is In Its Standard State

0 Comments